Alternaria alternata
New immunotherapy treatment with Alternaria Alternata (Alt a 1)
High Diagnostic Efficacy
Diagnosis by prick test with purified Alt a 1 allergen
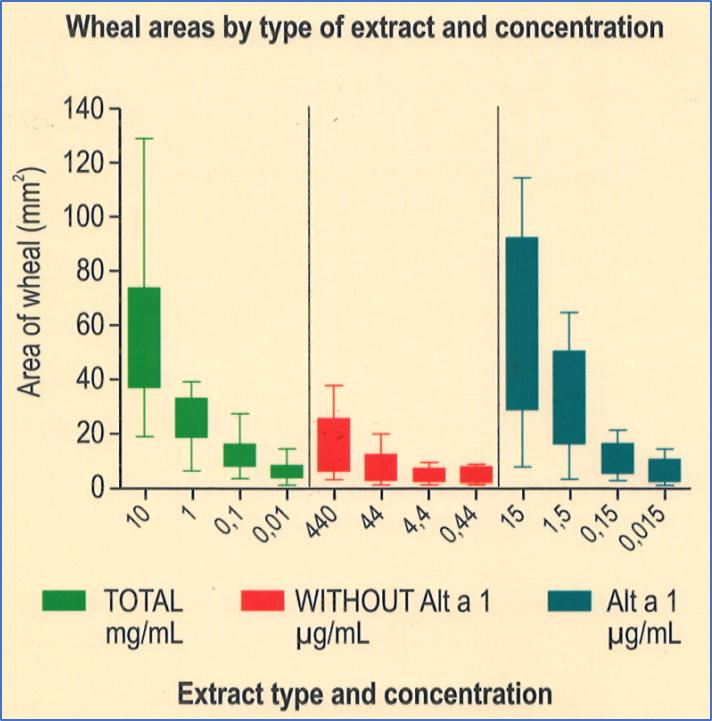
The wheal areas obtained with Alt a 1 protein are significantly greater than with extract of total Alternaria alternata and extract without Alt a 1.
Alternaria alternata
Alt a 1 is recommended for diagnosis and treatment of Alternaria alternata-sensitised patients. The major Alternaria alternata allergen is responsible for sensitisation of >90% of the allergic population.
Characteristics of purified Alt a 1
- Homogeneous product
- Expressed in units of mass
- Biologically standardised
- Allergen-specific immunotherapy
- Absence of possible sensitisations to other allergens
SDS Page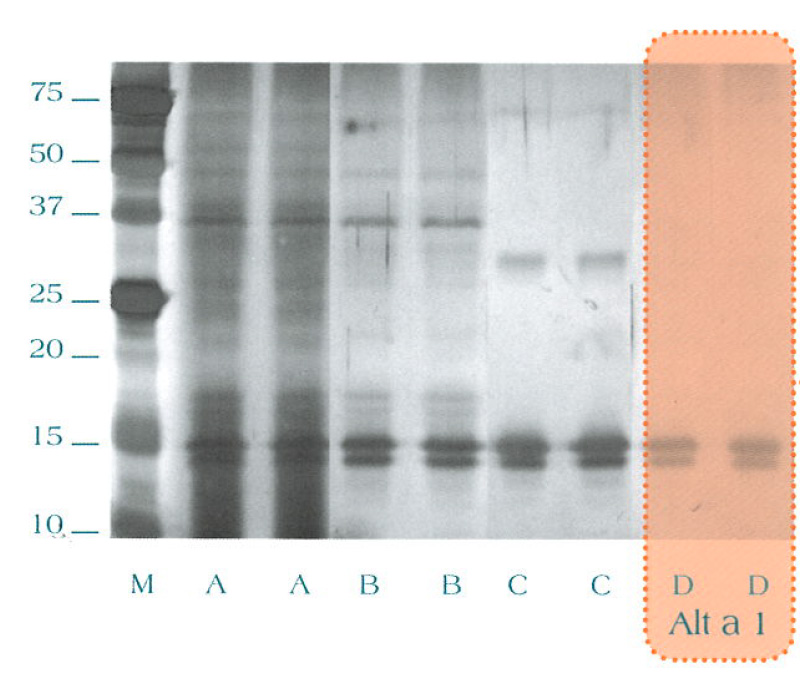
- Lane M: Molecular weight pattern
- Lane A: Complete Alternaria alternata extract
- Lane B: Alternaria alternata proteins purified by anion-exchange chromatography
- Lane C: Alternaria alternata proteins purified by cation-exchange chromatography
- Lane D: Alternaria alternata proteins purified by gel-filtration chromatography (Alt a 1)
Skin Test
Partial and preliminary results of Phase II/III Clinical Trials with Alt a 1
Clinical trial
- Multicentre, double-blind, randomised, placebo-controlled, parallel-group, clinical trail to evaluate the clinical efficacy and safety of immunotherapy with purified major allergen Alt a 1 in patients with allergic rhinoconjenctivitis with or without mild to moderate asthma, sensitised to fungus Alternaria alternata.
- The clinical trial is performed in three arms over 23 participating sites: 2 active at 0.25μg/mL and 0.458μg/mL, and a placebo.
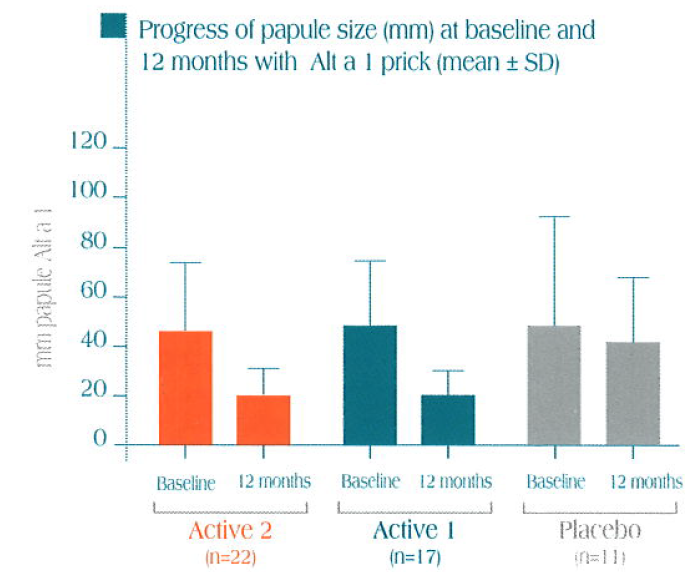
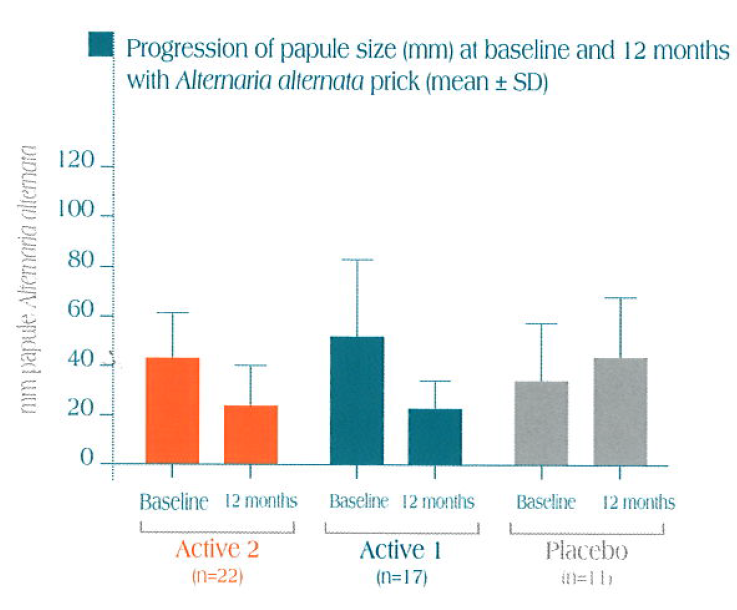
A statistically significant decrease in papule size is obtained after one year of immunotherapy with Alt a 1, both with Alt a 1 and with Alternaria alternata versus the control group.
Skin tests performed with Alt a 1 and Alternaria alternata versus control
Partial results collected after 1 year of treatment in 50 patients patients
Ig E Results
Active vs. control groups
Specific sIgE serum levels vs. Alt a 1 in the three treatment groups (mean ± SEM)
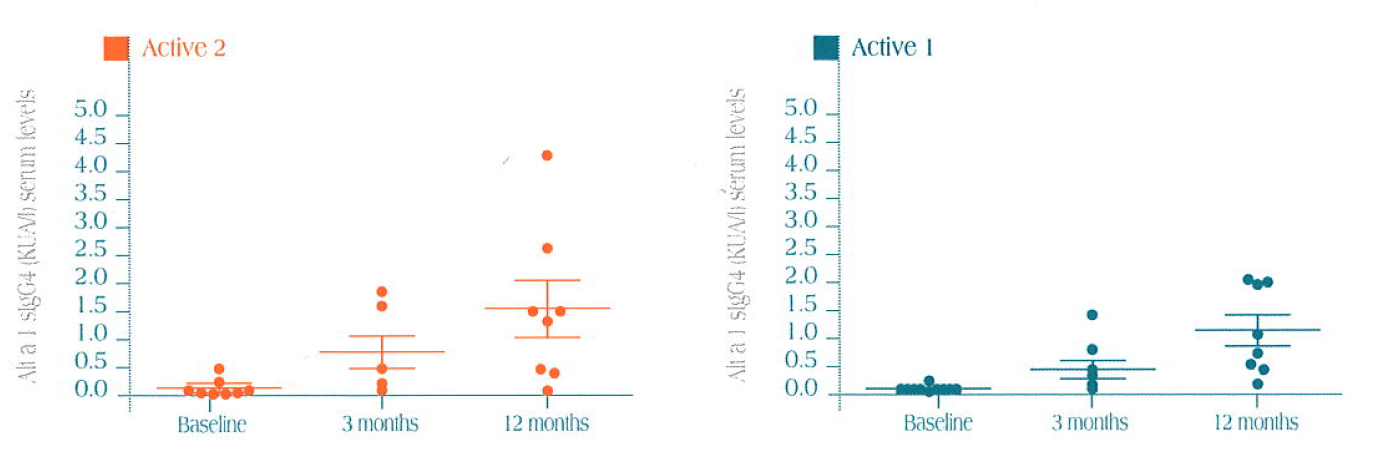
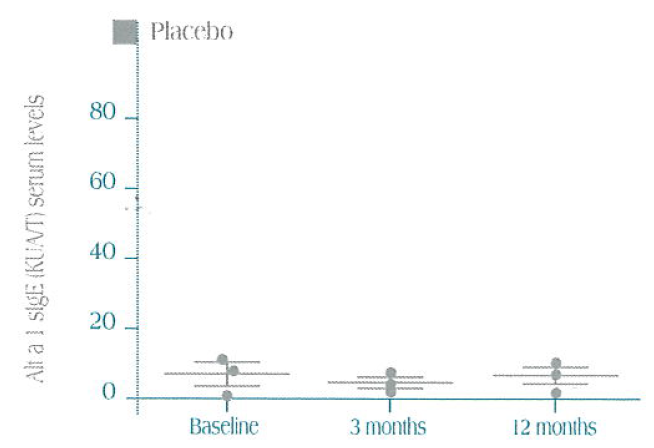
Statistically significant decrease in sIgE at 12 months treatment
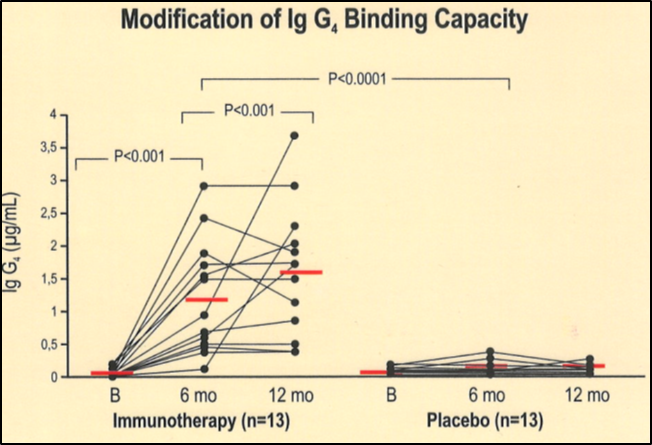
IgG 4 Results
Active vs. control groups
Specific sIgG4 serum levels in Alt a 1 in the three treatment groups (median ± SEM)
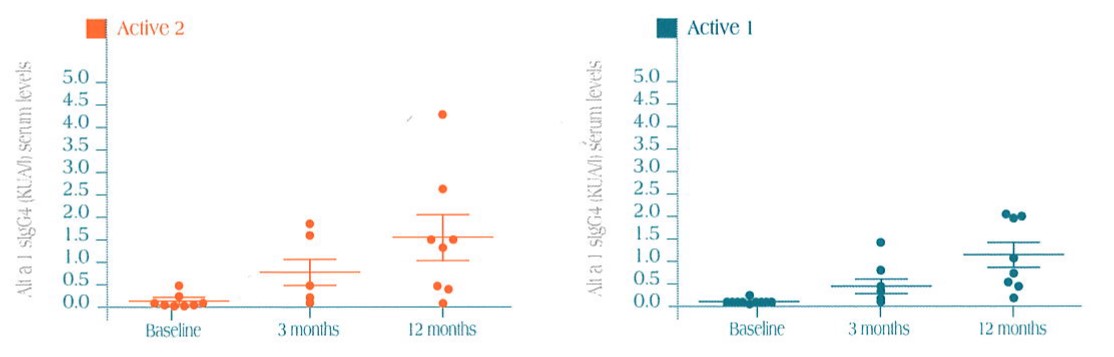
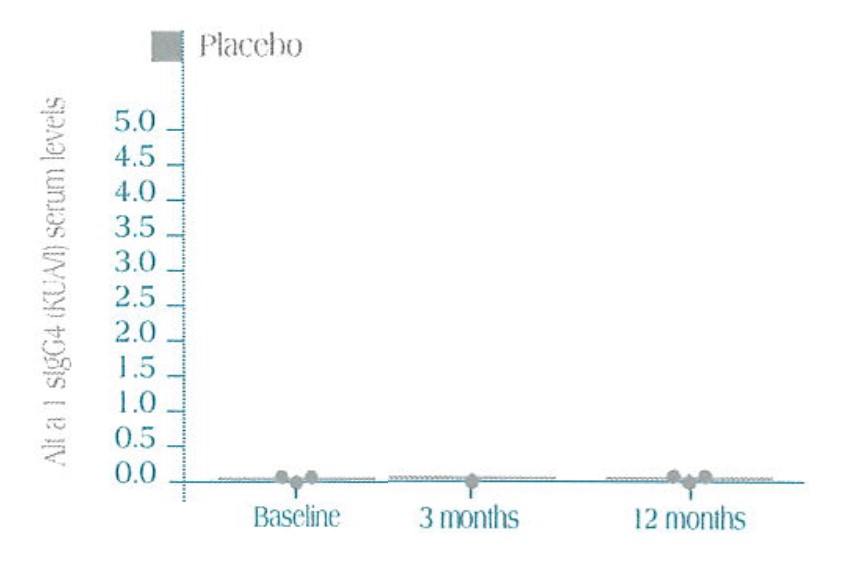
After one year of immunotherapy, a statistically significant increase in IgG 4 immunoglobulin is seen both in the Active 1 and the Active 2 versus the control group (placebo), where variation in the IgG 4 serum levels was not seen.
Safe and Well-tolerated
Safety
Alt a 1 is a safe treatment with a high level of tolerance; only mild or moderate adverse events were seen. No serious adverse events were reported. Previous clinical trials showed the same safety pattern.
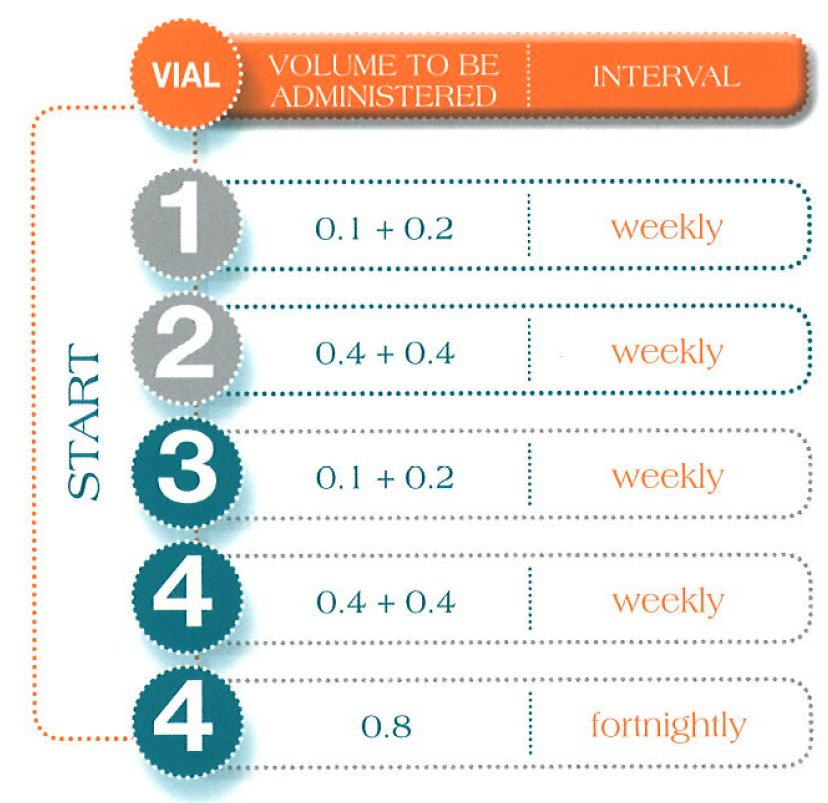
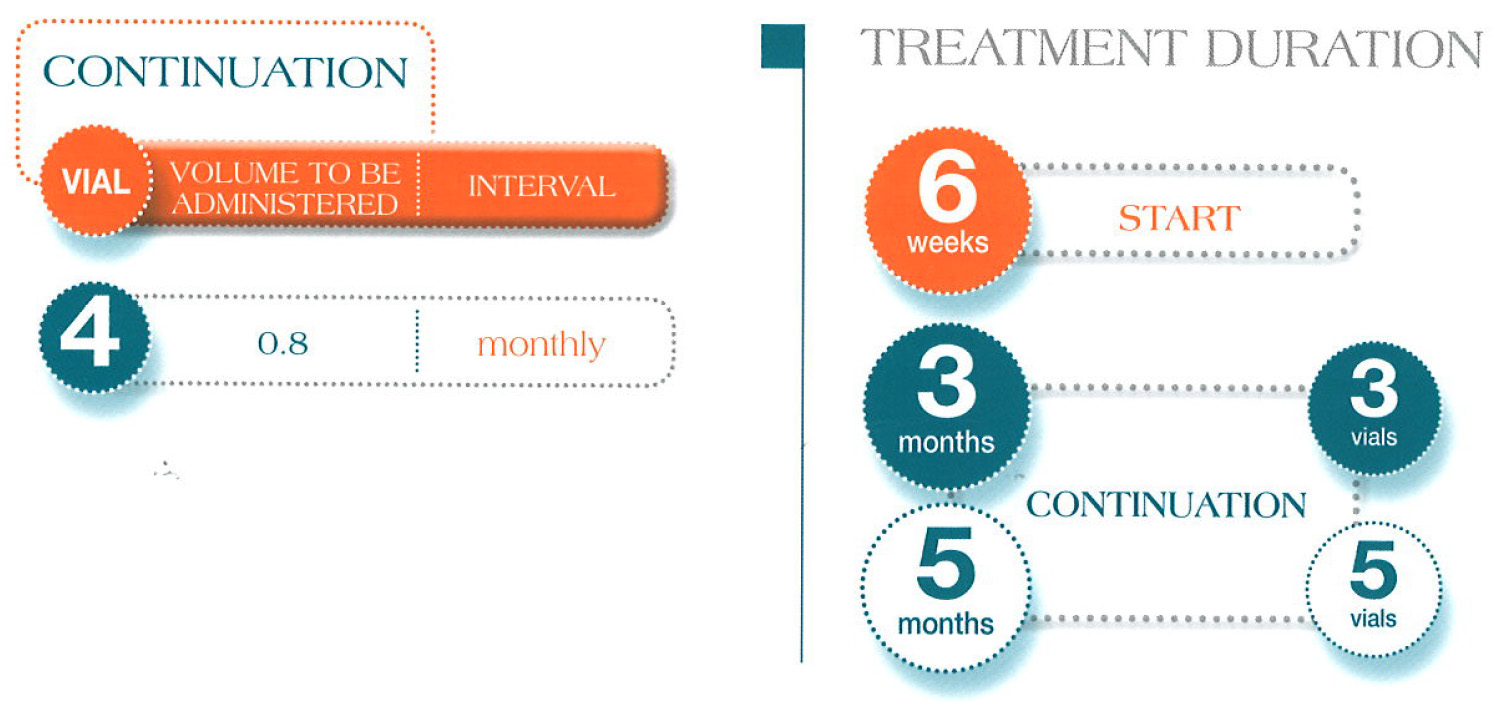
Administration Guidelines